Q1 Newsletter 2024
Q1 Newsletter 2024

President’s Message
With the first quarter of 2024 behind us, the positive outlook that emanates from our industry leaders suggests renewed optimism for the coming months. The annual J.P. Morgan conference kicked off in San Francisco to start the year as it normally does, and we were delighted to welcome more than 90 Life Sciences PA executives to a reception we co-hosted at JPM with Goodwin, CFGI, and five other state associations. During the event we could sense a buoyancy on the changing tide among our colleagues, and a hopefulness that our members once again will see breakthrough success in FDA approvals and designations, funding, and growth. Back home in Pennsylvania, we also saw a renewed sense of coordination and coalescence within and between the commonwealth’s life sciences ecosystems.
Life Sciences PA shared this enthusiasm, planning a robust calendar of events and initiatives to capitalize on the mindset we are seeing from our members. In February, LSPA members in the Lehigh Valley gathered at connect@Lafayette College to network with one another. In early March we gathered in Philadelphia for Meet the Leaders, a panel featuring women leading in the industry who discussed their journeys and offered actionable advice for emerging leaders.
Life Sciences PA also participated in an event in Harrisburg recognizing Pennsylvania Rare Disease Day to help bring awareness to necessary legislation that assists in patient access and treatments for thousands of rare disease patients across the commonwealth. Rare Disease Day provided another opportunity for LSPA to remind lawmakers there are still thousands of diseases affecting millions of patients around the world – including in Pennsylvania – who struggle with unmet medical needs and who are praying for our scientists to deliver solutions.
In preparation for the Annual Dinner & Showcase April 10 in Philadelphia, we hosted the 2024 Annual Awards honorees as guests on the Being a Life Sciences Leader podcast throughout February and March to give insights into their exceptional achievements and how they help lead the innovation ecosystem in Pennsylvania.
We are thrilled to see a renewed optimism within our membership, and remarkable partnership statewide thanks to efforts by the Greater Philadelphia Chamber’s CEO Council for Growth, the Lehigh Valley Economic Development Corporation, and the Pittsburgh Life Sciences Alliance, which officially launched this week. We are proud to assist in facilitating strategic connections between key players in the industry, knowing that rising tides do indeed lift all boats.
This quarter’s edition of The Newsletter offers a glimpse into the work being performed by your trade association, Life Sciences PA. We invite you to provide feedback on The Newsletter; feel free to send a note to any member of the LSPA team. We also invite you to visit the “Industry News” section of our website to catch up on the progress of our life sciences community more broadly. If you would like to contribute your news to the “Industry News” section, please let us know.
Thank you for your continued support.
All the best,
Christopher Molineaux
President & CEO
Life Sciences Pennsylvania
Life Sciences PA SUPPORTS THE LAUNCH OF THE keystone lifesci collaborative
march 6, 2024
Leaders from the life science industry from across the Greater Philadelphia area gathered March 6 to launch an industry-driven, regional partnership committed to strengthening the region’s life sciences industry. This partnership is supported by a collaborative team of workforce development, economic development, and education partners including West Philadelphia Skills Initiative, Greater Philadelphia Chamber of Commerce, Philadelphia Works, Life Sciences PA, and others. It is supported, in part, by a federal “Good Jobs Challenge” grant from the US Economic Development Administration.
Advocacy
ADVOCACY ABTRACT
January 19, 2024
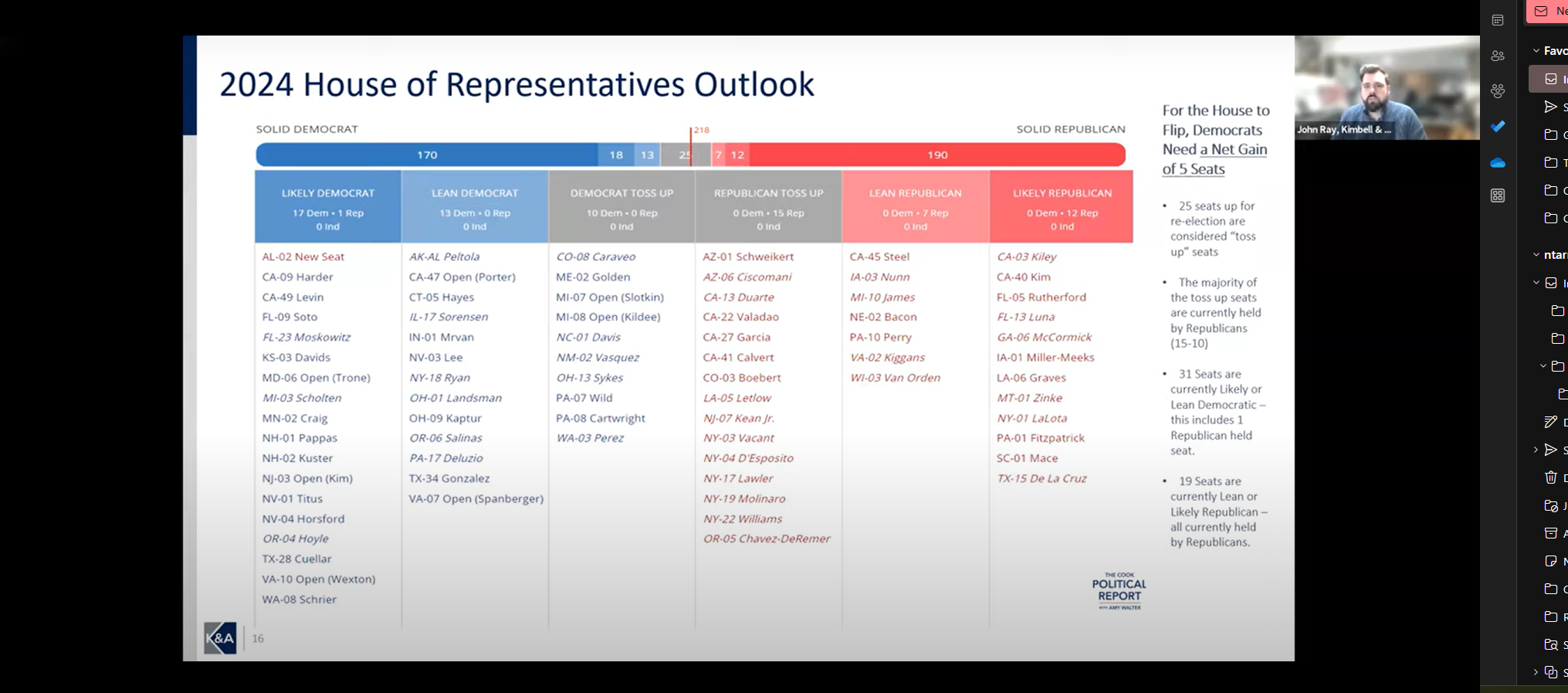
As we reached the one-year milestone of this program, experts Scott H. Mackenzie, Principal, Winning Strategies Washington and John Ray, Senior Director, Government Affairs, Kimbell Associates discussed implications for the next steps of the “laddered” approach to the Continuing Resolution for Government Funding. We also looked ahead to health policy changes advancing through Congress and in the Biden Administration & Must-Pass legislation and expectations for this election year.
PennsylvaniA RARE DISEASE DAY PRESS CONFERENCE AND LUNCHEON WITH LEGISLATORS
MARCH 18, 2024 | Harrisburg, PA
LSPA is honored to have co-hosted PA Rare Disease Day in the Pennsylvania Capitol with Pennsylvania Rare Disease Advisory Council (PARDAC). This press conference and lunch presented an important opportunity to raise the needs of Pennsylvania’s rare disease community. It was also a chance to acknowledge the wonderful work of so many of our member organizations researching and developing novel therapies and cures for patients with rare disorders. Rare disease patients throughout Pennsylvania are faced with unique, often life-threatening challenges every day. Of the nearly 10,000 rare diseases in the US, only a few hundred have any established treatment.


Advertisement

Advertisement

LSPA PRESIDENT & CEO Chris Molineaux JOINS CSBA AT THE NEW YORK STOCK EXCHANGE FOR Closing Bell
January 3, 2024
Life Sciences Pennsylvania President and CEO Chris Molineaux had the opportunity to sit down with the New York Stock Exchange for an interview, and later joined life science colleagues from across the country to ring the NYSE closing bell. We are thrilled to have joined leaders from our fellow life science trade organizations to kick off January as Biotech Month! Thank you to Jennifer Hawks Bland and NewYorkBIO for convening this group of leaders at the epicenter of the world’s financial sector and providing us the opportunity to recognize the national collaboration it takes to drive innovation and improve patients’ quality of life.

LSPA MARCH-IN LETTER SUBMITTED TO NIST
FEBRUARY 5, 2024
In December of 2023, The Biden Administration released a new plan focused on lowering healthcare and prescription drug costs for Americans. Among other proposals, this initiative will put forth “…a proposed framework for agencies on the exercise of march-in rights on taxpayer-funded drugs and other inventions, which specifies that price can be a factor in considering whether a drug is accessible to the public.”
Since the 1980 passage of the Bayh-Doyle Act, which significantly altered the patent and trademark landscape, presidential administrations of both political parties have understood that simply relicensing patents would disrupt the already fragile ecosystem of academic research institutions, industry partners and others. Unfortunately, these misguided proposals do not accurately account for the significant time and resources private investment organizations and companies make to research and develop safe, effective, and usable medicines. Although federal agencies have had the authority to do so, no agency has ever exercised march-in rights in the 40-years since the concept was included in Bayh-Dole.
Life Sciences PA will continue to ensure members of the Pennsylvania congressional delegation and other stakeholders understand how undermining the strong intellectual property protections we have in the U.S. will significantly disrupt an innovation ecosystem developing novel therapies and technologies for unmet medical needs.
PULSE COALITION FLY-IN
FEBRUARY 29, 2024
On February 29, PULSE partners from across the country met with policymakers on Capitol Hill to provide a real-world perspective on the critical role of pro-innovation mergers and acquisitions (M&A) and other collaborations in the life sciences industry.
These meetings came as policymakers at the Federal Trade Commission (FTC) and Department of Justice (DOJ) have increasingly looked to broadly deter M&A, without considering its fundamental role in supporting life sciences innovation.

EDA TECH HUB “PROPEL” PROPOSAL SUBMITTED
FEBRUARY 29, 2024
After being selected last October as one of thirty-one federal innovation and technology hubs, the Philadelphia region – led by the Ben Franklin Technology Partners of Southeastern Pennsylvania – submitted a grant application to the federal Economic Development Administration. This project entitled PROPEL – the National Center for Precision Medicine will build a globally competitive public-private partnership around the region’s collective life sciences ecosystem. It will leverage Philadelphia’s leadership in cell and gene therapy research to develop the supply chain, manufacturing and workforce assets necessary to drive innovation and growth in this key economic sector. Life Sciences PA is pleased to be a partner in this effort and is hopeful this application will receive the “Implementation Award” for this grant program.
SMTA FLY-IN
MARCH 5&6, 2024
Life Sciences PA participated in a national partner “Fly-In” where we met with the Offices of Rep. Mary Gay Scanlon, Rep. Summer Lee, of Rep. Lloyd Smucker, Rep. Brian Fitzpatrick as well as Rep. Madeline Dean and Rep. Dr. John Joyce to discuss the importance of HR 1691 as well as other legislative priorities of the Med Tech Community.
SPARK THERAPEUTICS “BREAKING BARRIERS” EVENT WITH CONGRESSWOMAN HOULAHAN
MARCH 14, 2024
Representative Chrissy Houlahan (D-PA) visited Spark Therapeutics to learn more about their gene therapy development and research. Congresswoman Houlahan also joined Spark CEO, Ron Philip, and other key staff for a tour of their facility. Congresswoman visited Spark Therapeutics in West Philadelphia as part of a Women Breaking Barriers discussion celebrating Women’s History Month. Spark Therapeutics, a member of the Roche Group, was founded in March 2013 as a result of the technology accumulated over two decades at Children’s Hospital of Philadelphia. Spark Therapeutics launched the first gene therapy for a genetic disease that has received regulatory approval in both the U.S. and EU. Congresswoman Houlahan joined women in biotech – including Lara Flynn from Life Sciences PA – and discussed the importance of STEM education to advancing innovation in healthcare and Pennsylvania’s continued role as a global leader in researching and developing new therapies, devices, and diagnostics to improve lives.

Life Sciences PA, HINJ, and DE BIO congressional staff reception
MARCH 21, 2024
As we kicked off the March College Basketball tournaments, Life Sciences PA, Healthcare Institute of New Jersey, and Delaware Bio created an opportunity in a casual setting to meet with congressional staff to explore connections to our industry and deepen relationships across party and state lines.

Economic Development Plan
January 30, 2024
Life Sciences PA is honored to have been invited to Governor Shapiro’s announcement January 30 of a comprehensive economic development plan for Pennsylvania, and thrilled that the Governor chose to hold this press event at Orasure Technologies in Bethlehem – a global diagnostics company and member of Life Sciences PA whose CEO, Carrie Manner presided over the event. It has been nearly 20 years since the Commonwealth last published such a sweeping plan, and this decision is a clear indicator the Governor is committed to incentivizing Pennsylvania’s innovation economy.
The intent to formulate this plan was initially announced in September 2023. The Governor’s administration worked closely with Life Sciences PA and many other stakeholders and partners to obtain feedback on a long-term roadmap for innovation, economic growth, and opportunity. As representatives of a prominent industry in Pennsylvania, we know there is great potential in this type of initiative and are proud to see the life sciences listed as a “Priority Sector” in this proposal. You can read more about the announcement HERE and view the full plan HERE, but among items of interest to our members are the establishment of a Pennsylvania Innovation Fund, Industry and Higher Ed Innovation Councils, and a “Buy Pennsylvania” initiative.
Governor Shapiro’s Budget Address
FEBRUARY 6, 2024
Governor Shapiro presented his proposed budget for 2024-2025 in Harrisburg before a joint meeting of the State House and Senate. The plan calls for $48.3 billion in spending by the state government, which represents an 8 percent increase over last year’s budget. This proposal is the second by Governor Shapiro since taking office, and the full transcript of the Governor’s budget address can be found HERE.
This budget address comes on the heels of the Governor’s announcement the week prior of a comprehensive economic development plan for the Commonwealth. More information on that plan can be found HERE. The Governor’s budget includes $600 million in new and expanded funding to implement this plan. Included in that number is:
- $500 million for the PA SITES program to remediate and develop sites for business attraction.
- $20 million to support large-scale innovation and leverage Pennsylvania’s research and development assets
- $3.5 million to create and launch the Pennsylvania Regional Economic Competitiveness Challenge that will invest in the development of comprehensive strategies to propel regional growth.
In alignment with our state policy priorities, Pennsylvania’s three Life Sciences Greenhouses are funded at their annual level of $1 million each, for $3 million total, and the Research & Development and Keystone Innovation Zone Tax Credits are each fully funded. We are also pleased to see the funding for the Ben Franklin Technology Partners maintained at $17 million.
virtual manufacturing license
march 6, 2024
HB 2084 will establish a “Virtual Manufacturer License” in the Commonwealth of Pennsylvania. A virtual manufacturer is a company that sells their own prescription drug products and/or medical devices but contracts out the manufacturing and distribution operations. Virtual manufacturers never take possession of the product.
The Commonwealth of Pennsylvania currently issues a “Certificate of Record” for virtual manufacturers. The Certificate of Record is not a license, and therefore, Pennsylvania virtual manufacturers cannot distribute prescription drugs in states such as New York and Ohio, that do not accept certificates of record. This significantly limits the ability of these Pennsylvania companies to do business throughout the United States and puts them at a disadvantage when trying to distribute pharmaceutical or medical device products. Thank you to Representative Briggs for introducing this legislation and for the Governor’s Office of Transformation and Opportunity for their interest in this issue. We look forward to working with the Pennsylvania legislature to move this legislation forward.
OFFICE OF INTERNATIONAL BUSINESS DEVELOPMENT
march 21, 2024
Life Sciences PA is honored to have hosted a delegation of foreign trade and investment representatives from the Pennsylvania’s Office of International Business Development. These representatives are a critical link for Pennsylvania and for Pennsylvania companies as the commonwealth works to attract foreign investment and encourage Pennsylvania companies and organizations to develop relationships in various geographies around the world. This event served as an important opportunity to showcase the stakeholders that makeup Pennsylvania’s life sciences ecosystem and ensure these internationally-focused individuals have the information and first-hand view necessary to market the Commonwealth’s robust innovation economy to the rest of the world.

right to repair informational hearing
march 25, 2024
The Pennsylvania House of Representatives Committee on Commerce held an information hearing on the issue of “right to repair” March 25. Life Sciences PA provided written testimony to the Committee on this issue, which is particularly concerning for our medical technology member companies. Patient safety is of the utmost importance to our medical technology members. The devices and diagnostics our members bring to patients go through years of development and thorough review by the U.S. Food and Drug Administration (FDA) – the recognized gold standard, worldwide in technology review for safety and efficacy.
Medical devices, like most complex equipment, need periodic maintenance and repair. Proper servicing of these life-saving and life-sustaining devices is vital to their safe and effective functioning and the safety of patients and device users. As part of their commitment to patient safety, original equipment manufacturers (OEMs) dedicate extensive resources to establishing comprehensive servicing programs to ensure their devices are properly maintained and continue to meet safety and effectiveness requirements determined by FDA.
Legislation has and continues to be discussed in states throughout the country that would allow third-party repair servicers to attempt to repair these complex devices, and no longer rely on OEMs. Often times these proposals give unregulated third-party servicers unlimited access to service manuals and other proprietary OEM information and would serve to put patients and device users at greater risk. Access to the latest manuals is no substitute for the extensive training, knowledge, and expertise provided by the OEM. Life Sciences PA has serious concerns about any Right to Repair legislation in Pennsylvania that would allow third-party servicers to interfere with medical devices.
Advertisement

Advertisement
Events
OPEN HOUSE
January 23, 2024 | King of Prussia, PA
At the first quarter Open House, LSPA members gathered at the Life Sciences Center to network, connect, and see the benefits of utilizing the Center over their morning coffee. Join us at the next Open House, Wednesday, April 24, to build your life sciences network!

Connect @ Lafayette College & LEHIGH VALLEY ECONOMIC DEVELOPMENT COUNCIL
February 8, 2024 | LEHIGH VALLEY, PA
This complimentary, LSPA member-only networking event provided an opportunity for attendees to connect with members of the life sciences community from Lehigh Valley and surrounding areas while enjoying a cocktail reception and hors d’oeuvres at the Rockwell Integrated Science Center.
Making the Most of Your Membership
February 27, 2024 | King of PRussia, PA
Any member looking to maximize their membership experience is encouraged to join Making the Most of Your Membership. This roundtable discussion is designed for new members, or new employees of member companies to learn ways to build and improve their life sciences network, and understand the robust benefits available through membership at Life Sciences PA.
Meet the Leaders – A Breakfast Panel
March 15, 2024 | PHILADELPHIA, PA
Attendees gathered in Philadelphia for a leadership discussion featuring panelists Cindy Reiss-Clark of West Pharmaceutical Services, Katie Reuther of Penn Health-Tech, and Sharon Willis of Integral Molecular. They discussed their journeys throughout the life sciences, leadership traits and advice, and participated in an interactive Q&A with the audience.

Referral Prospecting – A Nontraditional Approach to Building your Business
March 20, 2024 | King of Prussia, PA
In this half-day course designed to equip attendees with strategic gameplans on how to navigate networking events, attendees were introduced to a proven process for making quality introductions and building relationships that result in trust, support, and a commitment to one another’s success.


Operator’s Manual
Life Sciences PA continued the digital education series in 2024, with experts from legal, fundraising, and drug development providing invaluable advice to members on best practices for their day-to-day operations.
Legal Answers Workshop
 LSPA began 2024 with our digital series, Legal Answers Workshop. Attendees heard from Ballard Spahr experts, Leslie John and Jason Leckerman as they reviewed 2023 life sciences developments and discussed the outlook and considerations for 2024.
LSPA began 2024 with our digital series, Legal Answers Workshop. Attendees heard from Ballard Spahr experts, Leslie John and Jason Leckerman as they reviewed 2023 life sciences developments and discussed the outlook and considerations for 2024.
Phase to Phase: The Guide to Drug Development
 Sponsored by Thermo Fisher Scientific and PPD, this complimentary event featured experts that presented innovative strategies for Phase 2 clinical trial planning and execution.
Sponsored by Thermo Fisher Scientific and PPD, this complimentary event featured experts that presented innovative strategies for Phase 2 clinical trial planning and execution.
The Art of Fundraising
 This virtual program hosted by Danforth Advisors and moderated by Barb Carlin featured speakers Craig Carra of MOBILion Systems and Kimberly Minarovich of Argot Partners as they discussed how to leverage the positive energy coming back from the JPMorgan conference and best practices on how to keep investor relationships warm during all phases of capital fundraising.
This virtual program hosted by Danforth Advisors and moderated by Barb Carlin featured speakers Craig Carra of MOBILion Systems and Kimberly Minarovich of Argot Partners as they discussed how to leverage the positive energy coming back from the JPMorgan conference and best practices on how to keep investor relationships warm during all phases of capital fundraising.
Advertisement

Advertisement
BEING A LIFE SCIENCES LEADER PODCAST
The newest season of Being a Life Sciences Leader features Life Sciences PA’s 2024 Annual Awards Honorees. These companies and individuals are paving the way for life sciences innovation in the Commonwealth, and will be honored at the Annual Dinner & Showcase, April 10 in Philadelphia. Stream the episodes below to learn about each of the award honorees:
Welcome 2024 Q1 New Members!
AB Marketing Consulting
Action Medical Technologies
ALG BioPharm LLC Healthcare Consulting
Autosomal Dominant Optic Atrophy Association (ADOAA)
Auxilius
Charles J. Raymond Consulting LLC
Cyto PHL
electronRx
ES Talent Solutions
JM Search
Mispro Biotech Services
OPIS
Ori Biotech
Pathways Neuro Pharma Inc.
Paychex
PSC Biotech
Ryan’s Renegades
THEMA CORP
Thread Bioscience Inc.
Warren Avenue Investors
Annual Report Quick Links
Get Updates
from LSPA
Stay up-to-date on the latest news and events from Life Sciences PA, insights from the life sciences industry, and so much more!

Life Sciences Pennsylvania was founded in 1989 by a biotech scientist at Penn State University. Today it has grown to represent the entire life sciences industry – medical device companies, pharmaceutical companies, investment organizations, research institutions, and myriad service industries that support the life sciences in Pennsylvania.